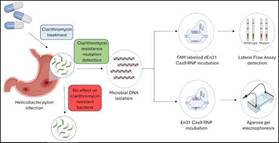
Helicobacter pylori (H. pylori) is a gram-negative bacterium that colonizes the human gastric epithelium, playing a pivotal role in the pathogenesis of various gastrointestinal disorders, including peptic ulcers and gastric cancer. Dyspeptic patients, particularly in resource-poor remote settings, face significant challenges in receiving timely and accurate diagnoses, primarily due to limited access to advanced diagnostic facilities and treatments. Recent advancements in molecular biology and diagnostic technology offer promising new methods for detecting H. pylori and its mutations, which could greatly benefit these underserved populations.
The traditional methods for H. pylori detection, such as endoscopy with biopsy or urea breath tests, necessitate sophisticated equipment and trained personnel, rendering them impractical in low-resource settings. Conversely, emerging techniques, such as polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP), provide rapid and cost-effective alternatives that do not require extensive laboratory infrastructure. These methods enable the direct detection of H. pylori DNA from clinical samples, thereby enhancing the speed of diagnosis.
Moreover, the identification of specific mutations within H. pylori strains is crucial, especially in the context of antibiotic resistance. In resource-limited environments, the use of antibiotics without prior resistance testing can contribute to treatment failure and the spread of resistant strains. By integrating mutation detection into the diagnostic process, healthcare providers can tailor antibiotic regimens more effectively, improving patient outcomes and reducing the burden of antimicrobial resistance.
The implementation of these innovative detection methods in remote settings can significantly enhance the management of dyspeptic patients. Improved diagnosis facilitates timely intervention, thereby preventing complications associated with untreated H. pylori infections. Furthermore, it empowers healthcare practitioners to implement targeted treatment strategies that adhere to local resistance patterns, optimizing therapeutic efficacy while minimizing adverse effects.
In conclusion, the advent of new diagnostic techniques for H. pylori detection and mutation analysis presents a valuable opportunity to enhance healthcare delivery for dyspeptic patients residing in resource-poor remote settings. By ensuring that these populations receive accurate diagnoses and appropriate treatments, we can alleviate the burden of H. pylori-related diseases and contribute to the broader goal of equitable healthcare access.